Recent research from the University of Texas at Austin sheds light on the crucial role of electrode materials in powering energy conversion and storage devices.
Transition metal oxyhydroxides, key players in technologies like water electrolyzers, alkaline batteries, and capacitors, undergo a process known as "in situ transition metal incorporation." This phenomenon, where trace metal impurities in the electrolyte replace transition metals within the oxyhydroxide structure, significantly impacts the performance of these materials.
A collaborative effort involving research groups led by Mullins, Resasco, and Milliron, alongside scientists from the Texas Materials Institute—Andrei Dolocan, Hugo Celio, and Xun Zhan—has produced groundbreaking insights into this process. Published in the Journal of Energy & Environmental Science, their study employed a wide range of characterization techniques to investigate the chemical and structural effects of in situ metal incorporation.
The researchers found that even minute concentrations of trace iron and cobalt cations in the electrolyte can infiltrate nickel oxyhydroxides, triggering a reconstruction of the material into an interstratified structure. This incorporation primarily occurs on the surface of the nickel oxyhydroxide films, as revealed by time-of-flight secondary ion mass spectrometry (TOF-SIMS) analysis.
Moreover, X-ray photoelectron spectroscopy (XPS) analysis showed that cobalt incorporation enhances the electronic conductivity of the material, while iron incorporation has a slight reducing effect. Electron energy loss spectroscopy (EELS) measurements suggest that incorporating both foreign metal cations alters the electronic environment of nickel centers through electron-withdrawing effects.
These findings align with electrochemical performance trends, demonstrating that cobalt incorporation improves energy storage capabilities, while iron incorporation enhances catalytic efficiency for water oxidation. By deepening our understanding of in situ metal incorporation, this study highlights its significance in shaping the surface chemical composition and structure of electrode materials, thus propelling advancements in electrochemical energy devices.
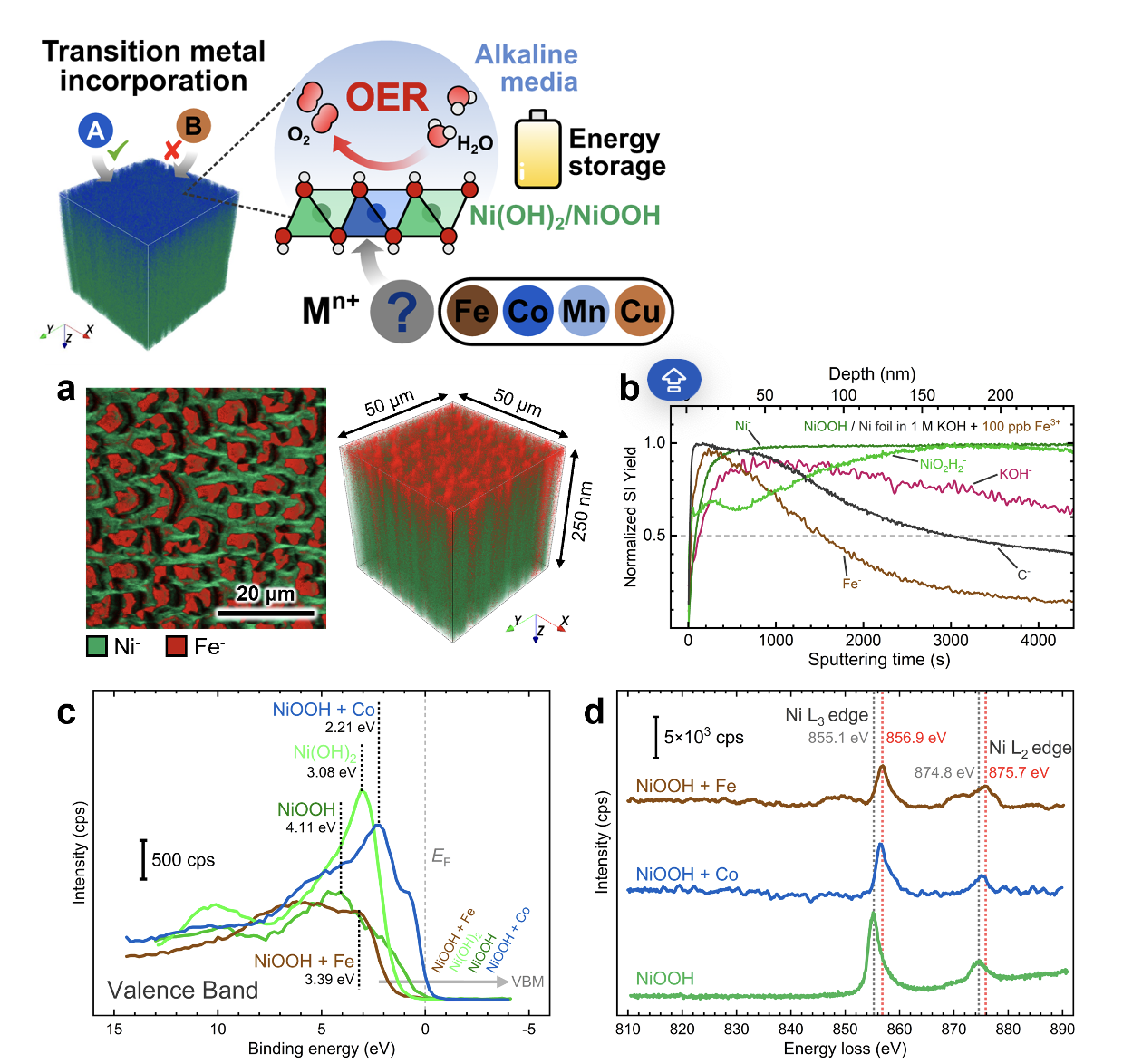
Figure description. TOF-SIMS characterization of nickel oxyhydroxide films after testing: (a) high-resolution 2D maps and 3D depth profile renderings showing the spatial distribution of Ni− and Fe− markers and (b) depth profiles (normalized to maximum) of secondary-ion fragments acquired from electrodes exposed to 100 ppb of Fe3+; (c) XPS valence band spectra for nickel oxyhydroxide electrodes exposed to Fe and Co impurities; (d) EELS spectra showing the energy loss shift of the Ni L3 and L2 edges with Co and Fe incorporation.


