All-solid-state batteries (ASSBs) are widely considered as the "Beyond Li Ion" technology, being potentially much safer and with much higher energy than commercial LIBs. ASSBs employ high voltage cathodes such as LiNi0.8Mn0.1Co0.1O2 (NMC811) and LiNi0.5Mn1.5O4 and a non-flammable inorganic separator termed solid-state electrolyte (SSE). For most ASSB architectures, a relatively thick metallurgically-rolled lithium foil is employed as the battery anode. However, limiting the amount of lithium is essential to achieving ASSBs with the targeted energy.
In fact, "Anode-Free" all solid-state batteries (AF-ASSBs) can deliver a 30% higher energy density than cells with a foil, and a game-changing 50% higher energy than the same cells employing standard graphite anodes. Unfortunately, depositing lithium directly onto a blank copper current collector (AF-ASSB) involves a series of challenges not encountered either with a foil or with graphite. Achieving stable cycling behavior in an AF-ASSB is a tremendous challenge due to the ongoing loss of lithium through several parasitic processes. Until this work, there have been very few reports of functional AF-ASSBs.
Existing commercial solid-state electrolytes are argyrodite (e.g. Li6PS5Cl, LPSCl) and doped lithium lanthanum zirconium oxide (e.g. LLZTO). Both inorganic electrolytes possess liquid-like ionic conductivity but have significant drawbacks, including poor resistance to dendrite-driven short circuiting. This is what has largely prevented wide-range AF-ASSB deployment. Mitlin and his team utilized site-specific cryo-stage electron microscopy (cryo-EM) to understand the origin of SSE failure and in parallel demonstrated how microstructural/interfacial design can enable state-of-the-art AF-ASSB performance. An important discovery reported was that to sustain electrochemical stability, control of lithium metal wetting on the empty current collector is key. By achieving uniform metal wetting by tuned collector surface structure, a stable argyrodite AF-ASSB is demonstrated for the first time. Mitlin's team also showed that mechanical milling-induced microstructural changes in the oxide electrolyte profoundly affect electrochemical stability, either providing or blocking the preferential paths for dendrite propagation. This work was done in close collaboration with Graeme Henkelman's and Donald Siegel’s modeling group at UT Austin, as well with PIs at CINT Sandia and at Purdue University.
These combined experimental - modeling studies are published as three nearly concurrent articles in leading journals and are sponsored by DOE Basic Energy Sciences. The publications are Dendrite Growth—Microstructure—Stress—Interrelations in Garnet Solid-State Electrolyte. Advanced Energy Materials. 2024, Journal Cover. Stable Anode‐Free All‐Solid‐State Lithium Battery through Tuned Metal Wetting on the Copper Current Collector. Advanced Materials. 2023, Journal Cover. Tuned Reactivity at the Lithium Metal–Argyrodite Solid State Electrolyte Interphase. Advanced Energy Materials. 2024. In sum, these findings have the potential to transform energy storage science by pointing the community to the role of rationally designed interfaces in enabling safe and reliable solid-state batteries.
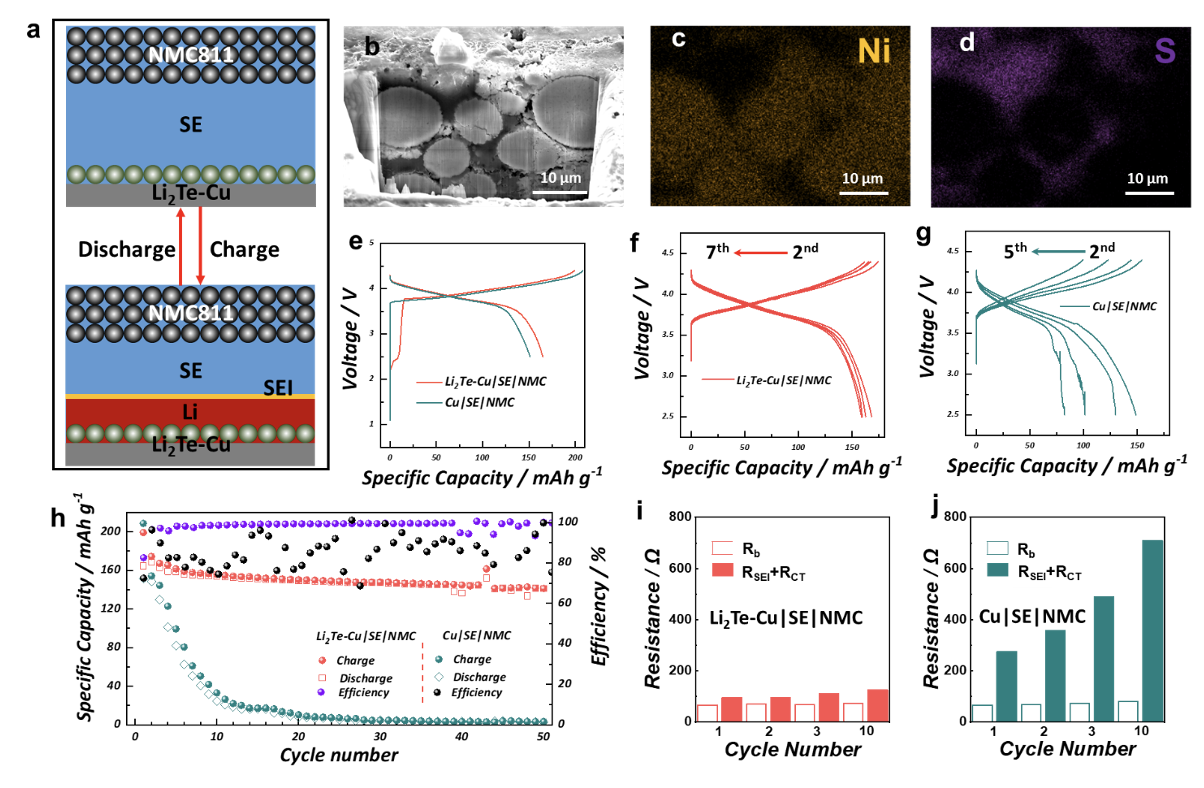
Figure 1. (a) Schematic diagram of the working principle in an anode-free solid-state battery. (b)-(d) Cryo-FIB SEM images and EDXS maps of the NMC cathode intermixed with the SE. (e)-(g) Galvanostatic charge/discharge profiles at 1st cycle, 2nd - 7th and 2nd - 5th cycle. (h) Cycling performance of Li2Te-Cu|SE|NMC and Cu|SE|NMC cells and (i, j) Results of the EIS analysis of the two specimens at different stages of cycling.

